Breakthrough innovations in cell encapsulation and immune tolerance to eliminate rejection
ImmunoShield's Mission
- Improve patient access to cell therapies
- Empower the development and application of cell therapies at scale
- Enable individuals to take charge of their health
- Fast Accelerated development
- Safe Reduced risk profile
- Easy Simplified application
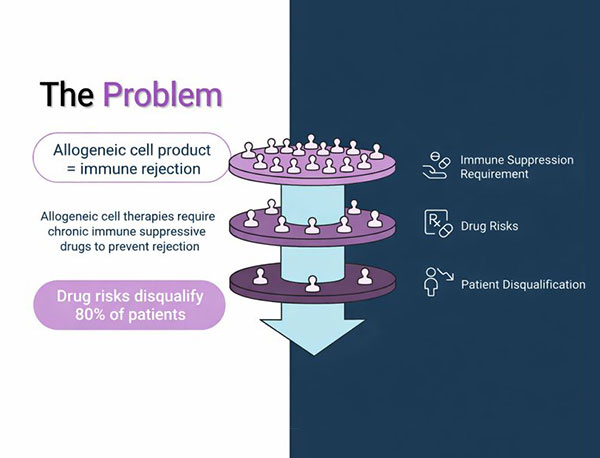
The Problem
Allogeneic cell therapies face significant barriers that limit patient access and market potential
-
Immune Rejection Risk Allogeneic cell products trigger immune rejection, requiring patients to undergo chronic immune suppression
-
Immunosuppressive Drug Risks Chronic immune suppressive drugs carry significant health risks and complications for patients
-
Critical Impact Drug risks disqualify 80% of patients This dramatically limits market size and patient access to life-saving therapies
The cell therapy market needs a solution that eliminates immune rejection
Without addressing this fundamental challenge, the promise of allogeneic cell therapies remains unrealized
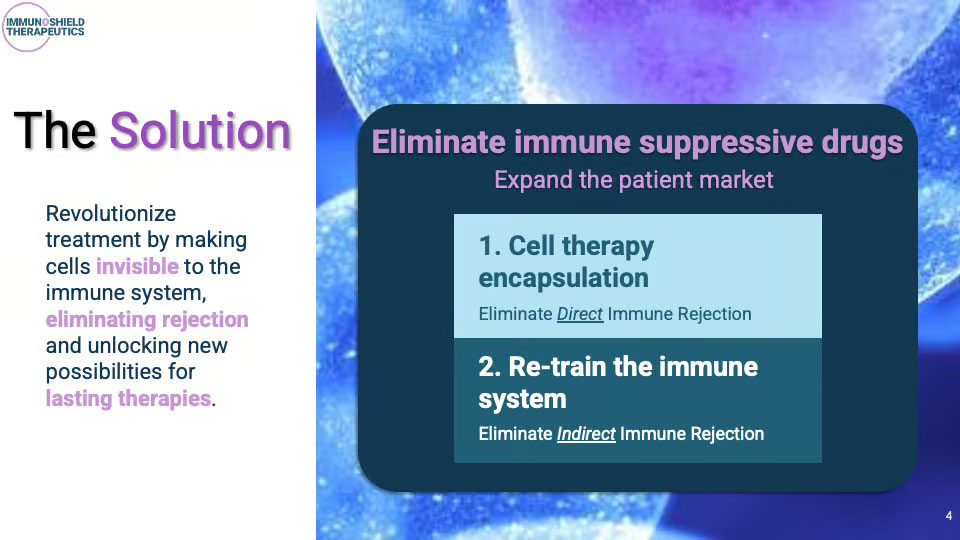
The Solution
Revolutionize treatment by making cells invisible to the immune system, eliminating rejection and unlocking new possibilities for lasting therapies
-
1
Innovative Spiral Macroencapsulation GeometryEliminate direct immune rejection
-
2
Re-train the immune systemEliminate indirect immune rejectionp
-
Eliminate immune suppressive drugs • Expand the patient market
ImmunoShield Innovation
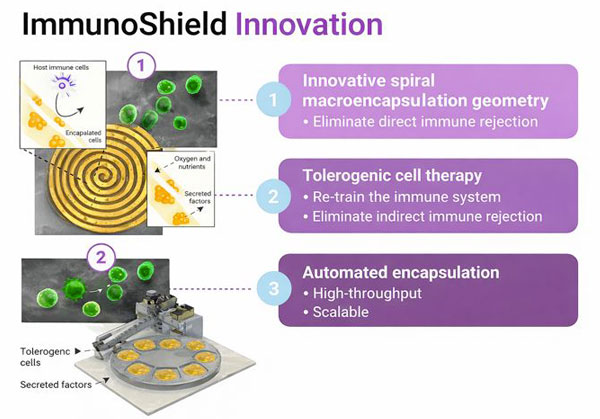
-
1
Innovative Spiral Macroencapsulation Geometry- Eliminate direct immune rejection
-
2
Tolerogenic Cell Therapy- Re-train the immune system
- Eliminate indirect immune rejection
-
3
Automated Encapsulation- High-throughput
- Scalable
Platform Benefits
-
Immune Protection
Proprietary technology shields allogeneic cells from immune rejection without compromising therapeutic function
- No immunosuppression needed
- Maintained cell efficacy
- Proven safety profile
-
Scalable Platform
Platform technology applicable across multiple cell therapy modalities and indications
- Broad applicability
- Streamlined manufacturing
- Cost-effective production
-
Market Expansion
Unlock the full patient population by eliminating the primary barrier to cell therapy access
- 5x market expansion
- Accelerated adoption
- Enhanced patient outcomes
Transforming Cell Therapy Economics
-
80%+
Previously ineligible patients now accessible
-
$50B+
Addressable market opportunity
-
3-5x
Potential revenue multiplier for partners
The Goal
Safe, functional cell therapy for every patient — with zero immune suppression
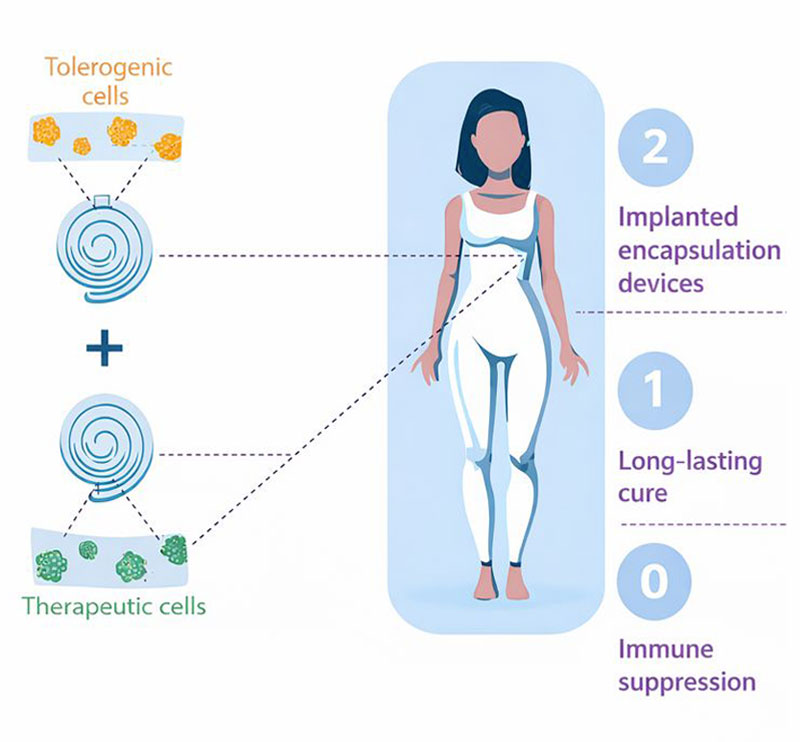
-
ImmunoShield Automated Encapsulation Technology
Macroencapsulation prevents direct immune rejection of the therapy
-
ImmunoShield Tolerogenic Cells
Tolerogenic cells prevent indirect immune rejection of the therapy
-
Partner's Allogeneic Cell Therapy
Allogeneic cell therapy provides a functional cure for the disease
-
Safe, functional cell therapy for every patient
-
2
Implanted encapsulation devices
-
1
Long-lasting cure
-
0
Immune suppression
-
Technical Advantages
-
Macroencapsulation Technology
- Innovative spiral geometry maximizes cell viability and nutrient exchange
- Physical barrier prevents direct immune cell contact
- Retrievable design allows for device removal if needed
- Scalable automated manufacturing process
-
Tolerogenic Cell Therapy
- Re-educates immune system to recognize cells as “self”
- Prevents indirect immune rejection pathways
- Eliminates need for chronic immunosuppressive drugs
- Long-term immune tolerance without systemic effects
-
Manufacturing Scalability
- High-throughput automated encapsulation system
- Quality control integrated into manufacturing process
- Cost-effective production at clinical scale
- Compatible with GMP manufacturing standards
-
Intellectual Property
- Multiple patent applications covering core technology
- Exclusive license from leading research institution
- Strong freedom-to-operate position
- Platform applicable across multiple indications