Explosive growth in cell therapy market with allogeneic therapies leading the charge
$18.3B
in cell therapies
18.7% CAGR
$1.5B
in allogeneic cell therapies
27% CAGR — Fastest growing segment
U.S. T1D Patients Eligible for Cell Therapy
Without immune suppression ~1,660,000 With immune suppression ~300,000
5.5x market expansion by eliminating immune suppression requirement
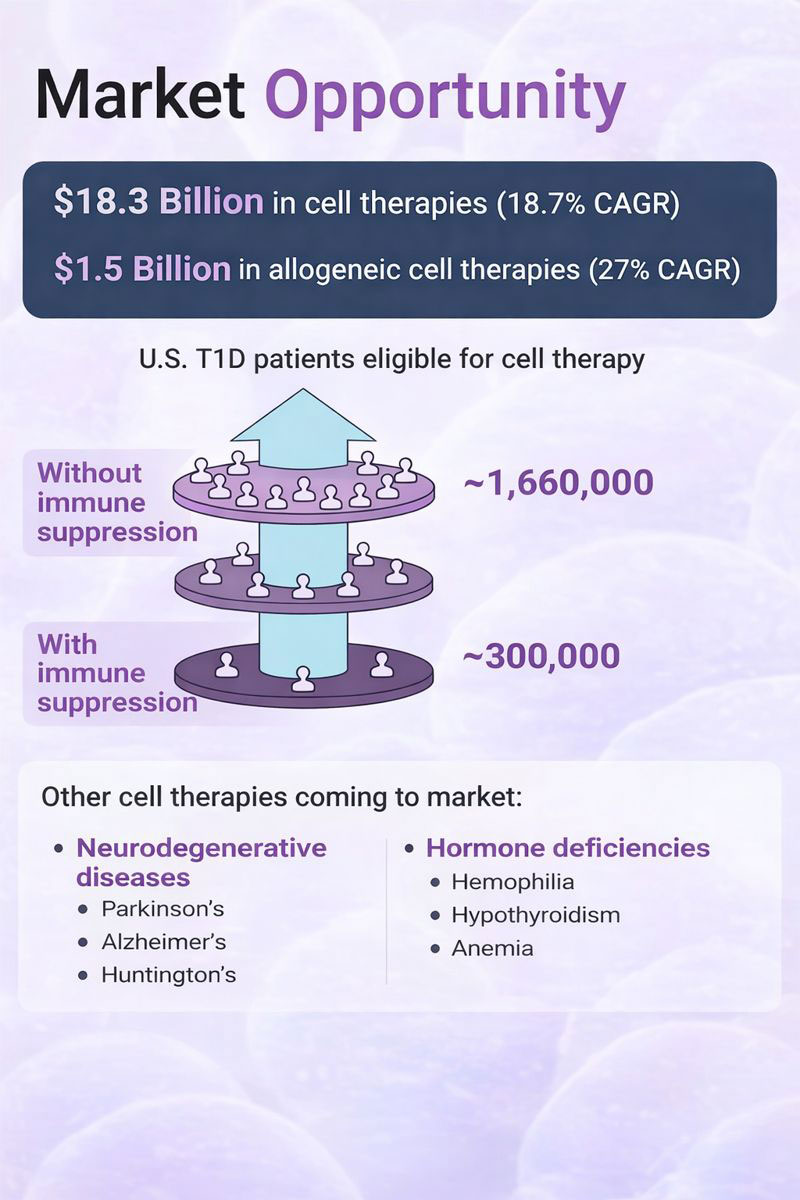
Other Cell Therapies Coming to Market
Neurodegenerative Diseases
- Parkinson’s
- Alzheimer’s
- Huntington’s
Hormone Deficiencies
- Hemophilia
- Hypothyroidism
- Anemia
ImmunoShield’s platform technology is disease-agnostic, positioning us to capture value across multiple high-growth therapeutic areas
Go-to-Market Strategy
Strategic path from technology development to market-ready product through pharma partnerships
-
1-2 High-Risk Technology
Early stage, uncertain future
-
Current Stage3-4 De-risk Technology
Non-dilutive funding and seed investors to complete independent pre-clinical studies
-
5-6 Pharma Partnership
Investment and co-development for a cell therapy product with ImmunoShield's platform to complete IND-enabling studies
-
7-8 Safety & Efficacy Trials
Partner company takes over manufacturing and advancement of co-developed product after IND submission, starts clinical trials, potential acquisition
-
9 Market Ready Product
FDA approved product ready to scale
-
Near-Term (12-18 months)
- Complete independent pre-clinical studies
- Validate automated encapsulation platform
- Secure pharma partnership for co-development
-
Mid-Term (18-36 months)
- Complete IND-enabling studies with partner
- Submit IND for first indication
- Establish manufacturing partnerships
-
Long-Term (3+ years)
- Initiate Phase I/II clinical trials
- Explore acquisition opportunities
- Expand platform to additional indications
Strategic Partnership Model
Our go-to-market strategy leverages pharma partnerships to accelerate development, reduce capital requirements, and maximize value through strategic acquisition
-
Lower Capital Need
Partner funds clinical development
-
Faster to Market
Leverage partner infrastructure
-
Higher Exit Value
Validated clinical data drives acquisition
Competitive Landscape
ImmunoShield's dual approach provides significant advantages over existing solutions
vs. Immunosuppressive Drugs
Current Standard of Care
- Severe side effects (infections, kidney damage, cancer risk)
- Excludes 80% of patient population
- Lifetime drug regimen required
- Significant ongoing costs
ImmunoShield Advantage
- No systemic immunosuppression needed
- Accessible to all patients
- One-time intervention
- Lower total cost of care
vs. Other Encapsulation Technologies
Existing Approaches
- Microencapsulation: difficult to retrieve, limited lifespan
- Traditional macrodevices: poor scalability
- Still vulnerable to indirect rejection
- Limited manufacturing throughput
ImmunoShield Advantage
- Retrievable spiral design
- Automated, scalable manufacturing
- Addresses both direct AND indirect rejection
- High-throughput production capability
vs. Gene Editing Approaches
Gene Editing Methods
- “Stealth cells” – complex manufacturing, uncertain longevity
- Higher regulatory hurdles
- Permanent genetic modifications
- Limited to specific cell types
ImmunoShield Advantage
- No genetic modification of therapeutic cells
- Clearer regulatory pathway
- Reversible intervention
- Platform applicable to any allogeneic cell type